Dr. Charul Bhanji
Technical Director
9821186618
© Copyright 2019. Regrow Biosciences Pvt Ltd. All Rights Reserved
FOR DOCTORS
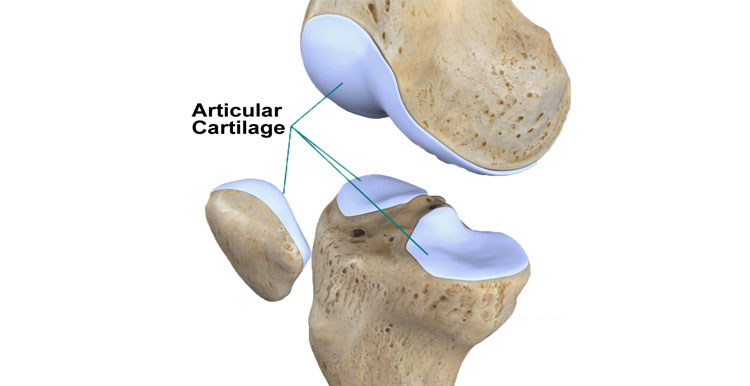
Articular cartilage is a highly specialized, uniquely designed biomaterial forming smooth gliding surface for diarthrodial joints.
There are three types of cartilage:
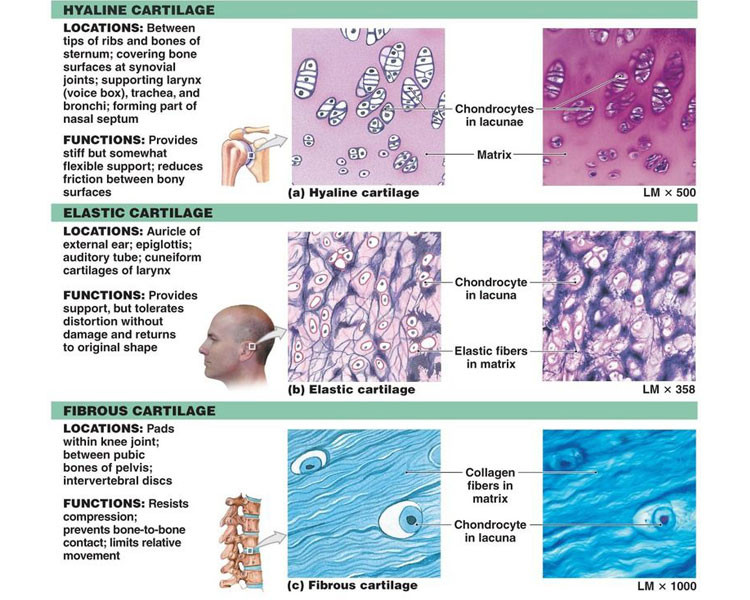

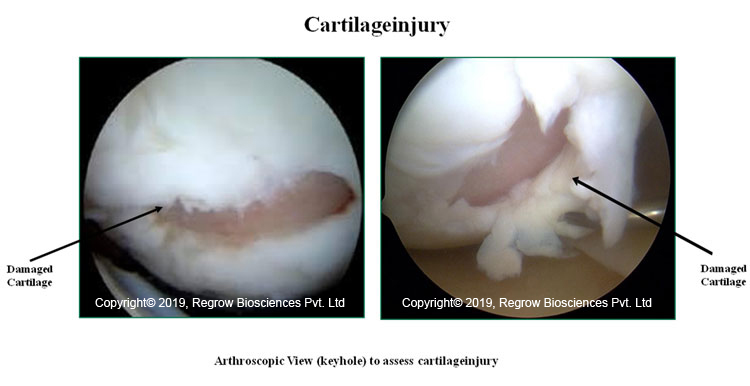
Cartilage has a poor blood supply unlike other tissue such as skin. Any injury to cartilage leads to underlying bones to rub against one another causing pain and inflammation.
Pain & swelling in affected joint
Typical 'clicking' sound
Stiff joints & sometimes locked joints
Restricted joint movement & rotation
Walking & climbing stairs becomes extremely painful
One has to discontinue jogging & sports
About Autologous Chondrocyte Implantation (ACI)
Cell therapies are in use for cartilage repair. ACI involves harvesting a biopsy of the patients cartilage, isolating & expanding the cells and re-implanting them into the defect site
History
Goal of Treatment
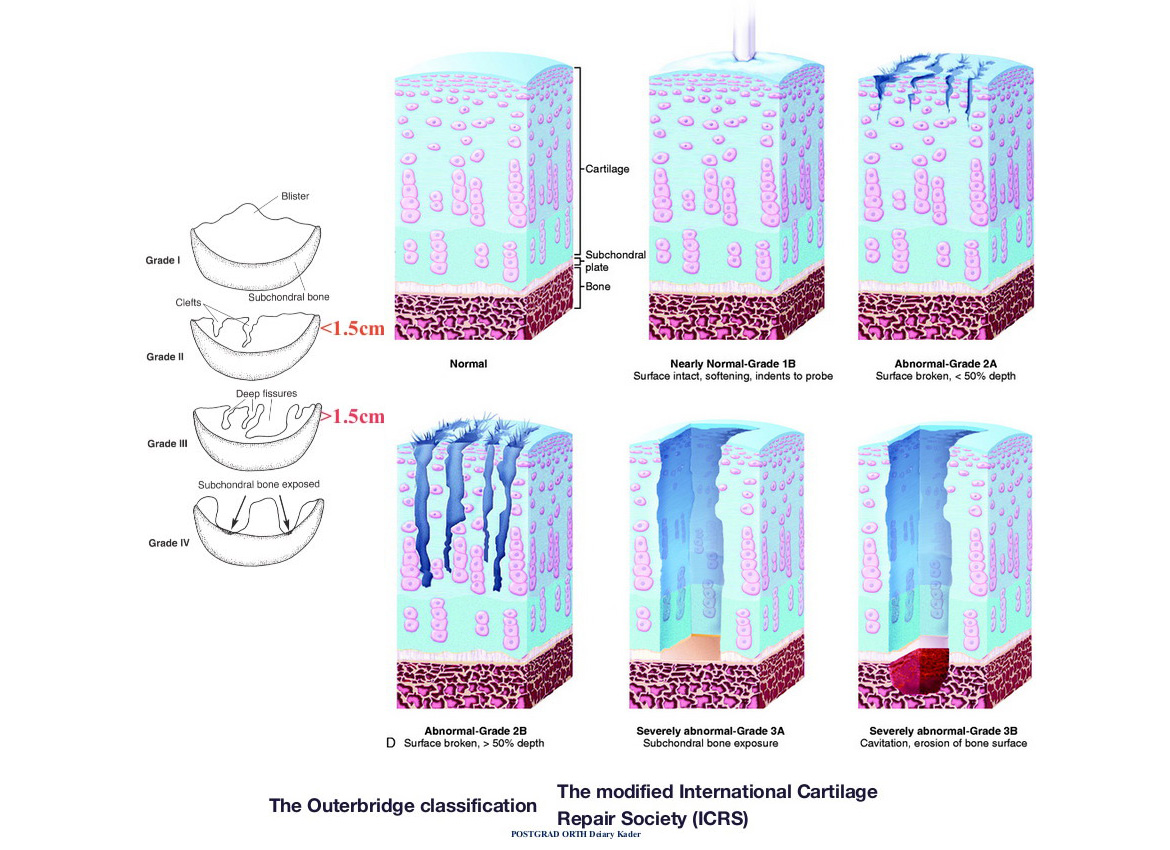
ICRS Grading for Cartilage Injuries
Unmet Clinical need of Orthobiologic treatment
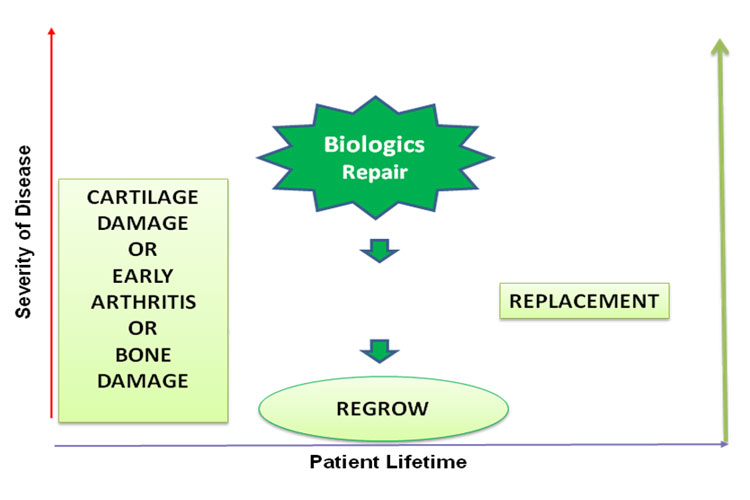
Biologic repair is now possible through Cell therapies such as Autologous Chondrocyte Implantation
Case Reports
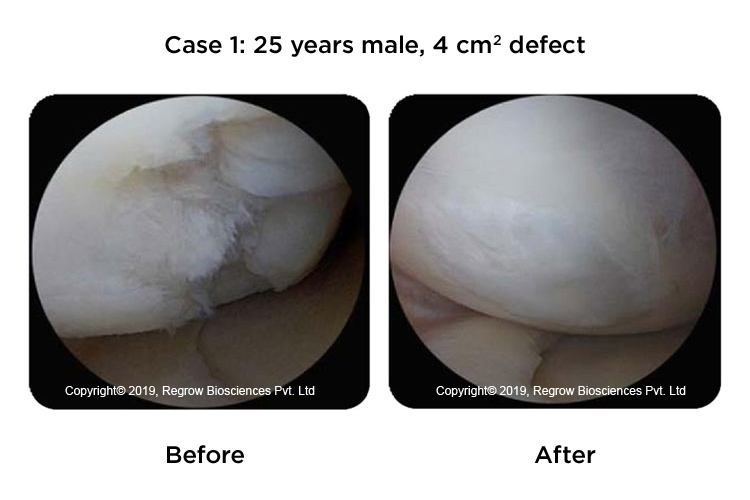
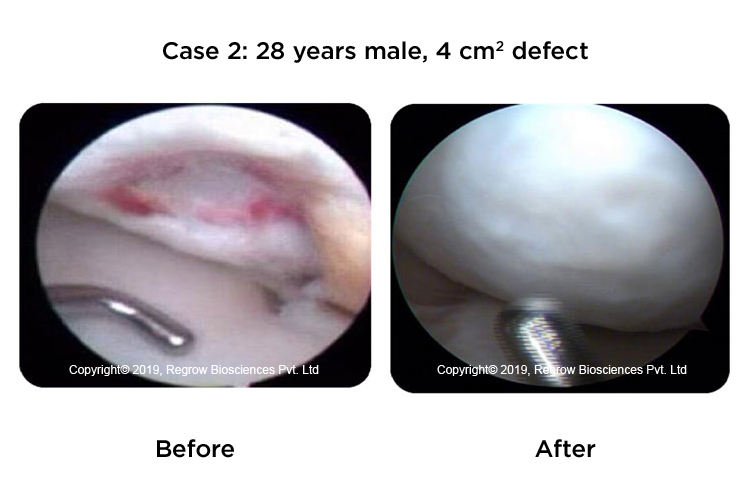
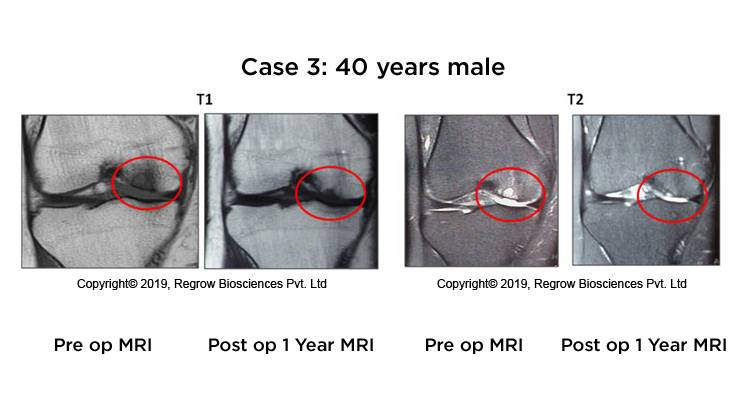
Our Process
Step 2
Cartilage Cell Implantation
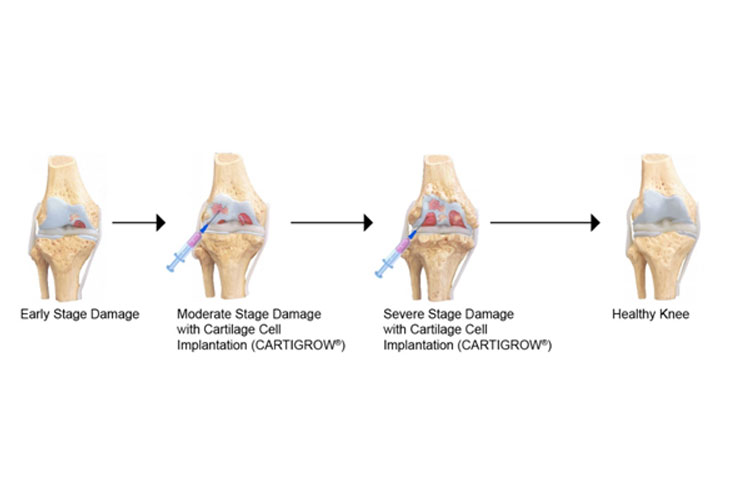
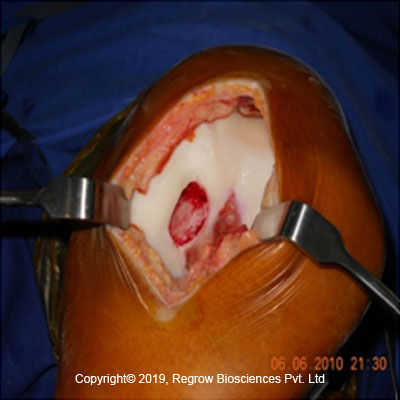
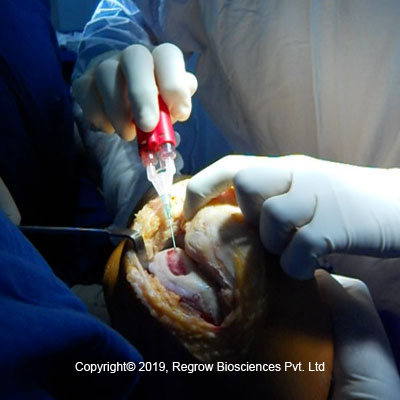
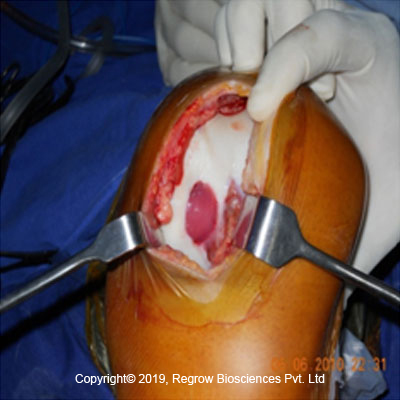
Implantation Procedure:
Step 1
Step 2
Step 3
Billing and Insurance
To place the order or to know more about the product, please call :
Dr. Charul Bhanji
Technical Director
9821186618
Mr. Amos Chopade
Medical Officer
9619835838
Medical Education
Please refer to our academic library for peer reviewed International Publications on ACI
Coming Soon...
Coming Soon